Fungicide resistance
Understand how fungicide resistance develops
Manage
disease pressure
Minimise
fungicide use
Engage
with extension
Fungicide resistance is a numbers game – higher disease pressure increases your chances of fungicide resistance developing.
Conducive weather conditions, virulent pathogens with a short life cycle, highly susceptible crop varieties and repeated use of fungicides all contribute to higher risk.
The risk also varies between different chemical groups and fungal pathogens. Specific strategies are recommended for situations considered to carry the highest risk.
To minimise risk, practice The Fungicide Resistance Five.
Advice for growers
Some simple management practices can help you reduce the risk of fungicide resistance occurring in your crops.
Select less susceptible varieties
Identify the most likely fungal threats for your region, especially where there is past evidence of fungicide resistance, and choose varieties that are less susceptible to these threats. Planting less susceptible varieties will reduce disease pressure, and your need for fungicide inputs.
Access regional variety guides:
Spray only if necessary and apply strategically
Fungicide use can select for resistance, so it is essential to only spray when the risk or presence of disease warrants it. Spray preventatively, at the first sign of disease, for best effect.
Rotate crops
A dynamic host environment makes it hard for the pathogen to adapt and keeps disease pressure low. Use time and distance to reduce disease carry-over.
Use non-chemical control methods
Most fungal pathogens require plant matter to survive, so eliminating the ‘green bridge’ and any infected stubble between seasons reduces disease carry-over, and thus disease pressure. Later or earlier sowing can also help reduce disease pressure, by changing the crop’s exposure to the pathogen.
ROTATE & MIX FUNGICIDE MoAs
Never apply the same fungicide twice in a row, and choose mixtures with different modes of action whenever possible. Avoid using Group 7 SDHI* and Group 11 QoI fungicides (seed dressing and foliar) more than once per season per crop.
*SDHIs applied as seed dressing/in-furrow can have differential foliar activity and will need to be assessed on a fungicide active by fungicide rotation strategy basis.
Managing fungicide resistance
Fungicide resistance is a preventable issue that can arise when fungi are overexposed to fungicide actives from the same chemical mode of action (MoA) group. It can become a major constraint to good disease control, especially where no alternative fungicide or effective host-plant resistance is available.
Fungicide resistance is an area-wide, social problem. Spores released by fungicide resistant fungi can spread over large areas in a short time. Misuse of fungicides and poor disease management practices on a single farm can affect everybody in the surrounding region.
The risk is greatest in situations of high disease pressure (influenced by conducive climate and agronomic practices), where there are susceptible crop varieties and virulent pathogens with a short life cycle, and fungicides with a single mode of action are used repeatedly.
Fungicide resistance management. Start with a strong foundation of less susceptible crop varieties, supported by integrated disease management, along with strategic and responsible use of fungicides.
Fungicide resistance evolution. Modified from CropLife Australia Fungicide Resistance Management Fact Sheet.
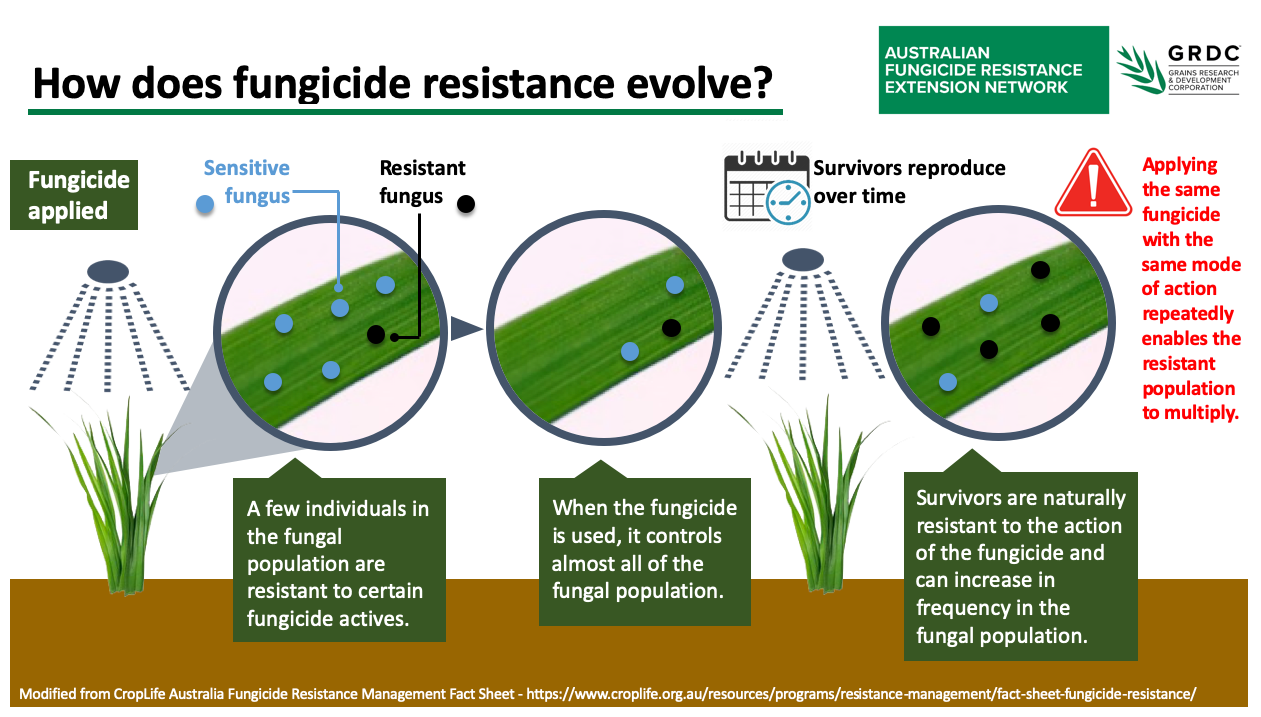
How does fungicide resistance evolve?
In any fungal population, there are likely to be individuals that are naturally less sensitive to particular fungicides or chemical mode of action (MoA) groups. When the fungal population is exposed to these fungicides, resistant individuals survive and continue to propagate.
After repeated use of the same fungicide or chemical active group, these resistant individuals come to dominate the fungal population and the effectiveness of the fungicide may be compromised in the field.
Ceasing use of a compromised fungicide or fungicide group can sometimes lead to a resurgence of the sensitive population and restored efficacy. But, this is hard to predict. In many cases, related fungicides within the same chemical group are likewise compromised, and will no longer provide effective control of that pathogen.
Key factors in fungicide resistance
The key factors in the ‘fungicide resistance equation’ are disease pressure and fungicide use.
Disease pressure is driven by multiple factors, including:
- conducive weather for the disease
- seasonal pathogen carry-over via stubble or the ‘green bridge’
- poor agronomic practices such as a lack of crop rotation
- poor hygiene
- planting of susceptible cultivars.
Fungicide use can select for resistance through repetitive, excessive or unnecessary use of the same chemical active compounds or related actives from the same mode of action group.
Fungicides by Modes of Action
Fungicides are grouped according to their biochemical mode of action (MoA). There are currently over 200 fungicides in 57 MoA groups that are approved for agricultural-use worldwide.
Very few of these chemicals are registered for use on Australian grains, which increases the risk of fungicide resistance developing within the industry.
(Remember, fungicides must be APVMA-approved for use on specific crops and can only be applied in compliance with the label directions.)
Dominant fungicide MoAs in Australia
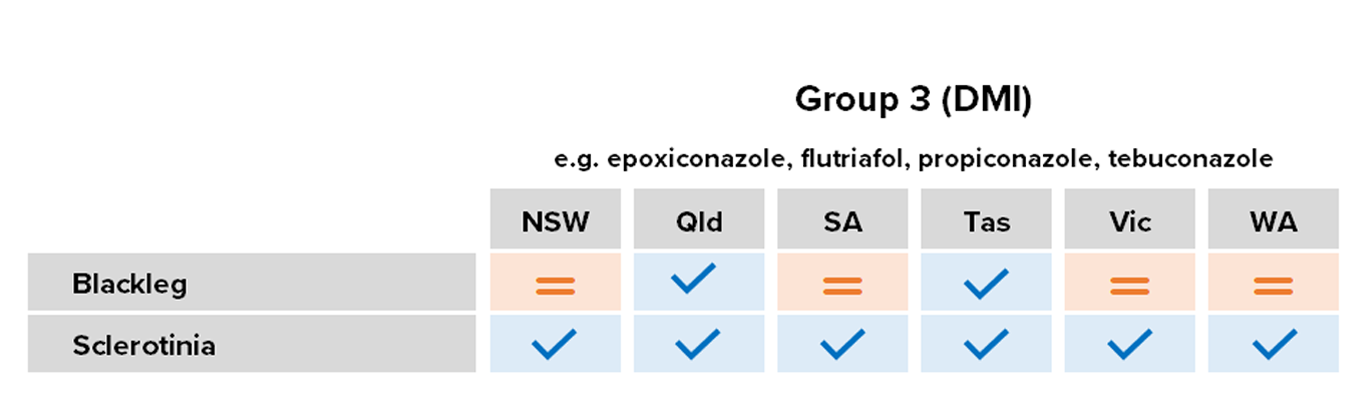
Group 3: Azoles/demethylase inhibitors (DMIs)
Common Actives
- Cyproconazole
- Epoxiconazole
- Flutriafol
- Propiconazole
- Prothioconazole
- Tebuconazole
- Triadimefon
Registered for
Most crops
Risk of fungicide resistance development
Moderate
Notes
The predominant group, they have generally been cheap and effective to use for many years.
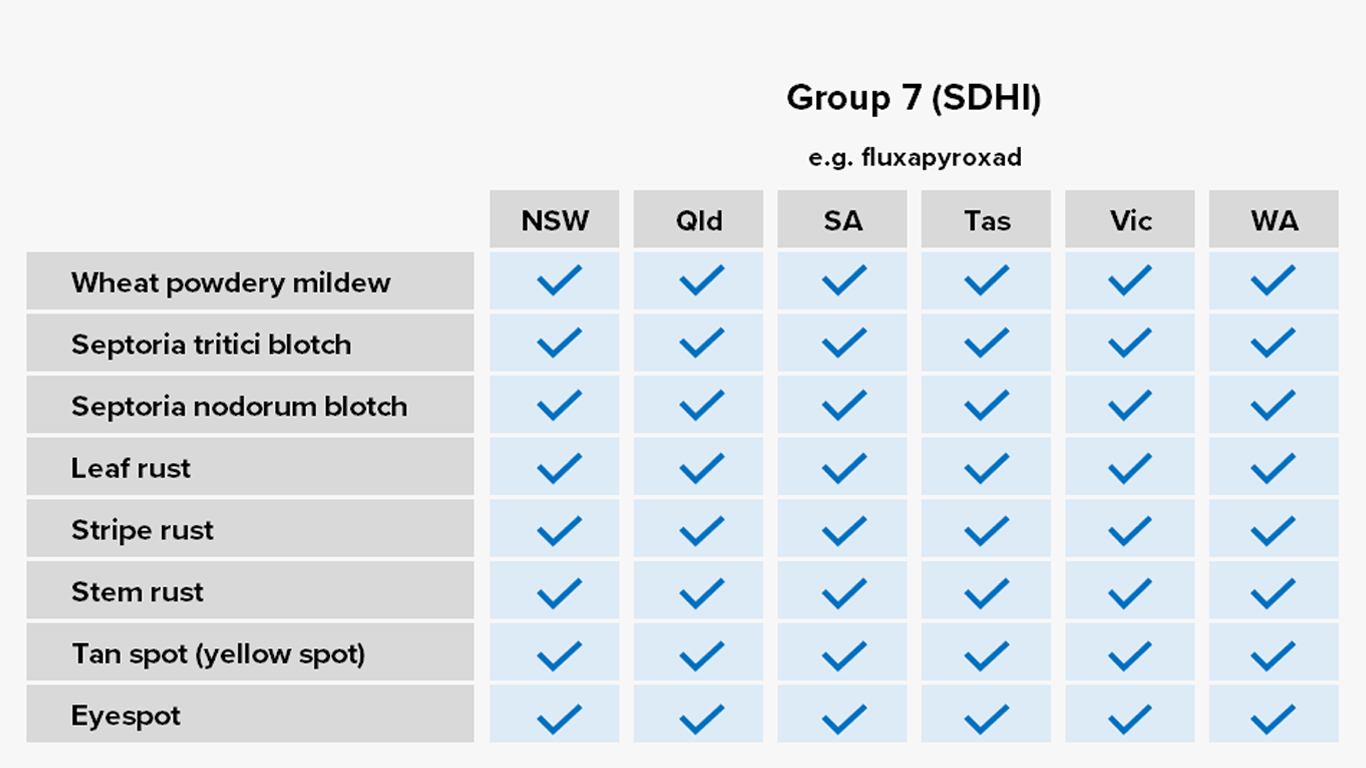
Group 7: Succinate dehydrogenase inhibitors (SDHIs)
Common Actives
- Bixafen
- Fluxapyroxad
- Penflufen
Registered For
Canola, cereals, winter legumes
Risk of fungicide resistance development
Moderate to high
Notes
Commonly distributed as seed dressings and mixing partners in some foliar formulations.
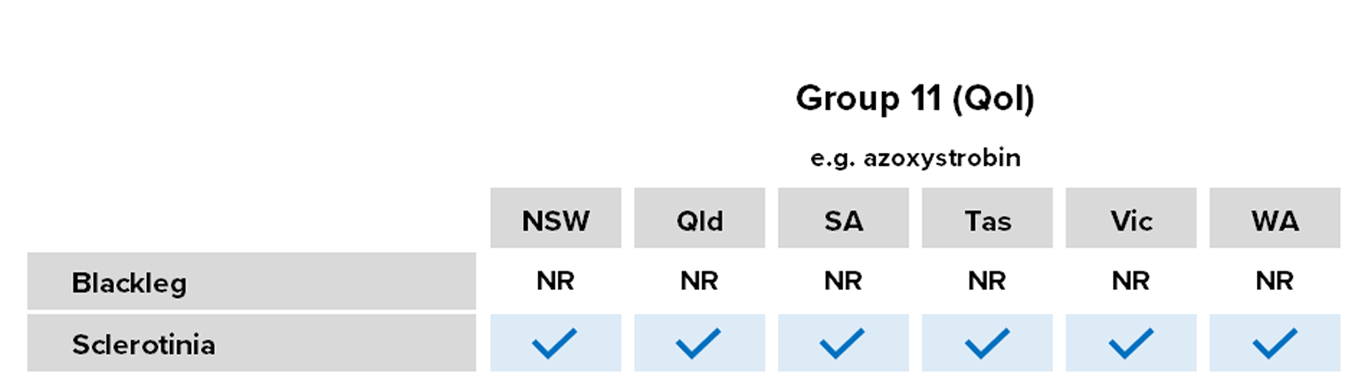
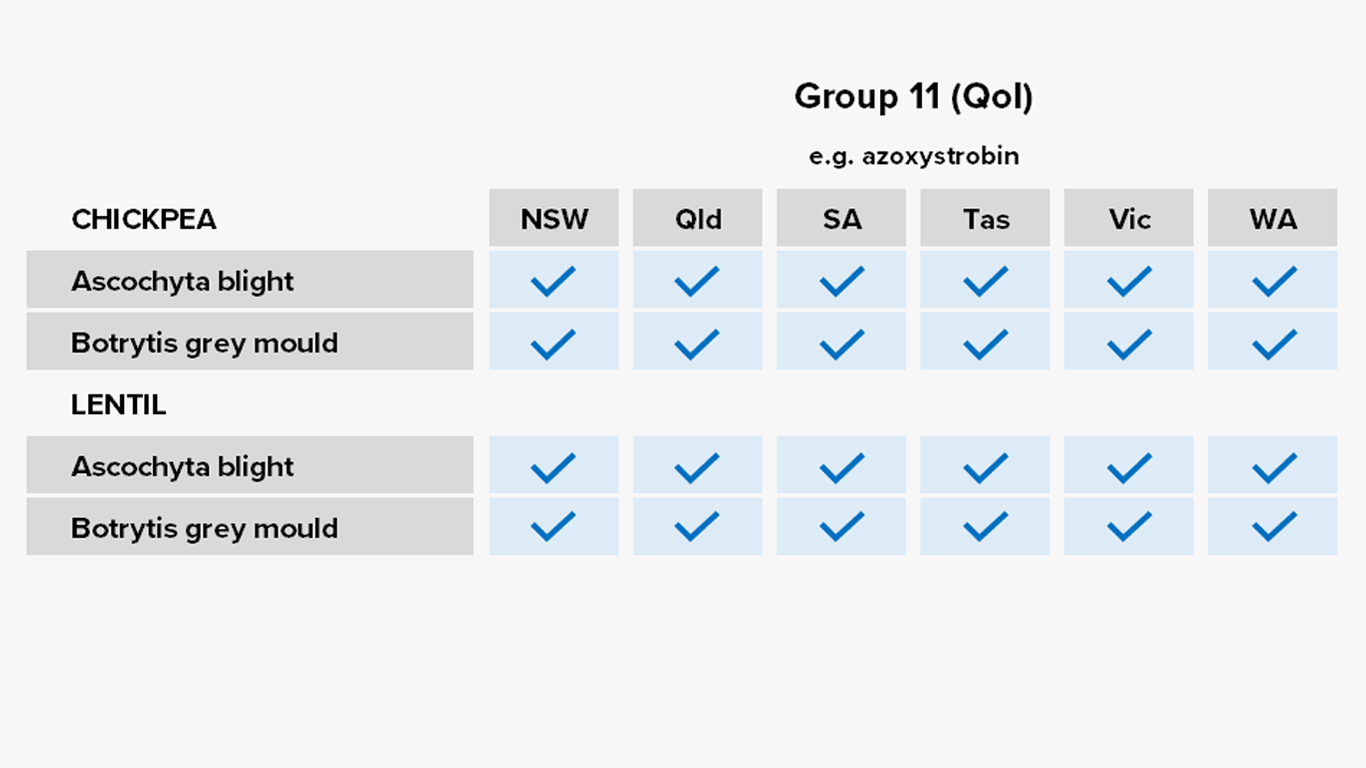
Group 11: Strobilurins/quinone outside inhibitors (QoIs)
Common Actives
- Azoxystrobin
- Pyraclostrobin
Registered For
Most crops
Risk of fungicide resistance development
High
Notes
Registered for some time, used as a mixing partner in some foliar and in-furrow formulations.
Other registered MoAs in Australia
Group 1: Methyl benzimidazole carbamates (MBCs)
Common actives
- Carbendazim
- Thiabendazole
Registered for
Pulses
Risk of fungicide resistance development
High
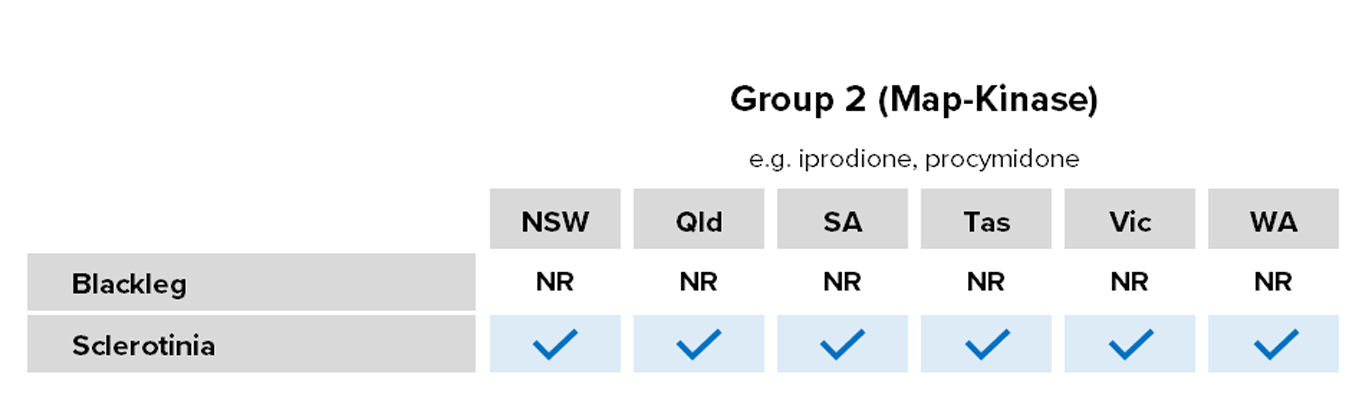
Group 2: Dicarboximides/MAP-kinase inhibitors
Common actives
- Iprodione
Registered for
- Canola (not for blackleg)
- Pulses (excluding chickpeas)
Risk of fungicide resistance development
Moderate
Group 4: Phenylamides/PAA
Common actives
- Metalaxyl
Registered for
Most crops
Notes
Not usually a principal fungicide for cereals, it is often used as a mixing partner to target oomycetes (e.g. Phytophthora spp., Pythium spp.)
Risk of fungicide resistance development
High
Group 5: Amines/Morpholines
Common actives
- Spiroxamine
Registered for
Barley (barley powdery mildew)
Risk of fungicide resistance development
Low to moderate
Group 12: Phenylpyrroles/PP fungicides
Common active
- Fludioxonil
Registered for
Canola, maize, peanut, sorghum
Risk of fungicide resistance development
Low to moderate
Group 13: Azanapthalene
Common active
- Quinoxyfen
Registered for
Barley (barley powdery mildew)
Risk of fungicide resistance development
Moderate
Group 14: Aromatic hydrocarbons and heteroaromatics
Common active
- Quintozene
Registered for
Peanut (soil borne fungi)
Risk of fungicide resistance development
Low to moderate
Group 33: Phosphonates
Common active
- Phosphorous acid
Registered for
Barley, canola, wheat
Risk of fungicide resistance development
Low
Notes
Principally used for the control of oomycetes (e.g. Phytophthora spp., Pythium spp.)
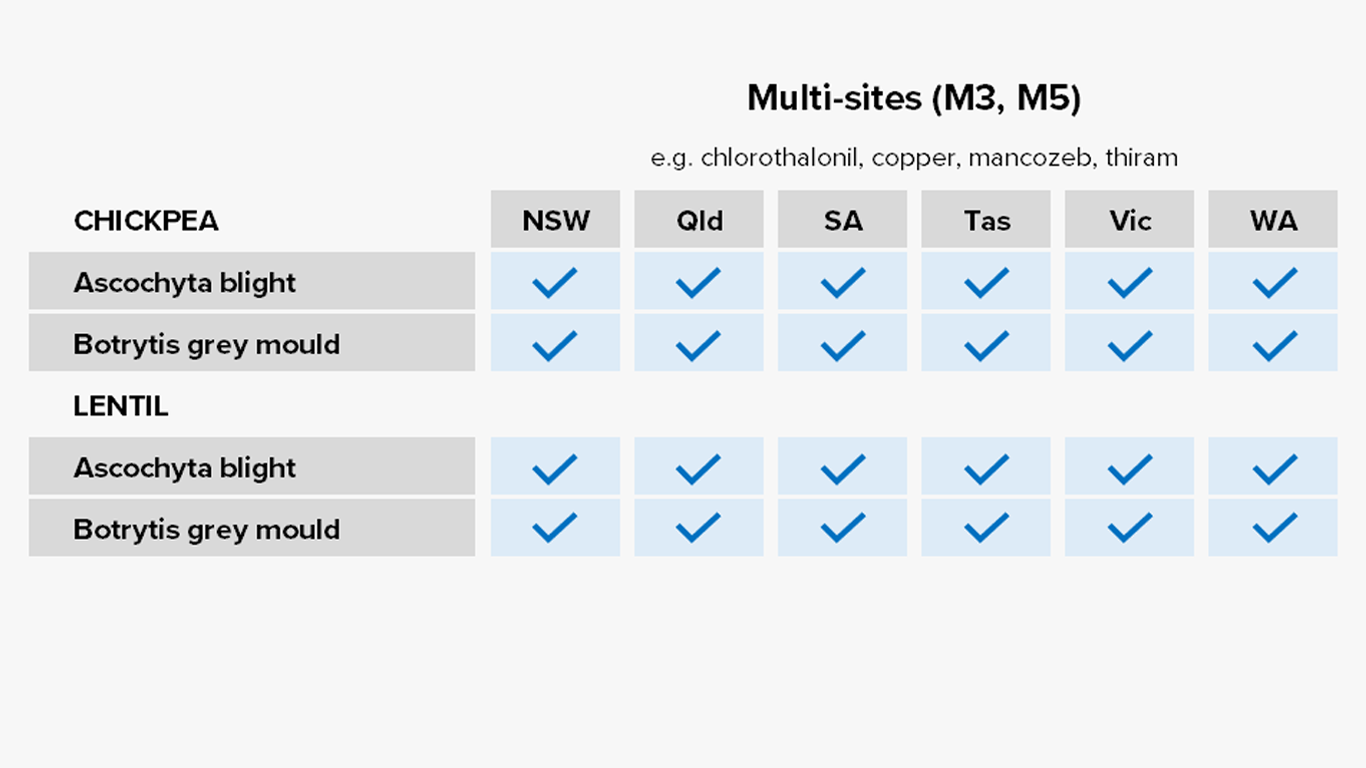
Group M1-M5: Multi-site activity
Common actives
- Copper
- Sulphur
- Mancozeb
Registered for
Predominantly pulses
Risk of fungicide resistance development
Low
Notes
Good rotation and mixing partner options for managing fungicide resistance.
Note: Fungicides are registered on a state/territory, crop, target pathogen, formulation and application rate basis. Current information on registered fungicides and their use can be found on the APVMA website at apvma.gov.au. Risk of resistance development indicated above is based on global experience and assessments by the Fungicide Resistance Action Committee (FRAC). Further information on MoA groups and risk of resistance development can be found on the FRAC website at frac.info.
Fungicide resistance in Australia
Multiple cases of fungicide resistance and reduced sensitivity have been identified in Australian grains crops since 2010. New cases have been reported regularly over the past decade, and more are expected to arise as survey and detection techniques become more sophisticated.
Fungicide resistance occurs when a previously effective fungicide fails to control a disease. It is a preventable issue, that can arise when fungi are exposed repeatedly to the same fungicide or fungicide actives from the same chemical Mode of Action (MoA) group. It can become a major constraint to good disease control, especially where no alternative fungicide or effective host-plant resistance is available.
Fungicide resistance terminology
Sensitive Fungi are considered sensitive when they are killed by a fungicide at recommended label rates.
Reduced sensitivity Fungi are considered as having reduced sensitivity to a fungicide when an application does not work optimally, but does not completely fail. In most cases, this would be related to small reductions in product performance, which may not be noticeable at the field level. In some cases, growers may find that they need to apply the maximum label rate of the fungicide to obtain the previously experienced level of control. Reduced sensitivity needs to be confirmed through specialised laboratory testing.
Resistant Resistance occurs when the fungicide fails to provide an acceptable level of control of the target pathogen in the field at the maximum label rate. Resistance must be confirmed with laboratory testing, and be clearly linked with an unacceptable loss of disease control when using the fungicide correctly in the field.
Lab detection Measurable differences in the sensitivity to the fungicide when the fungus is tested in vitro using tests recognised by the scientific community and/or detection of known or novel molecular mechanisms (e.g. genetic mutation, changes in target gene expression, etc.) of a fungal isolate. These changes can often be detected in the laboratory before any loss of fungicide efficacy is detected in the field. Lab detections are used to confirm reports of field resistance or reduced sensitivity, or to indicate the potential for resistance or reduced sensitivity to develop.
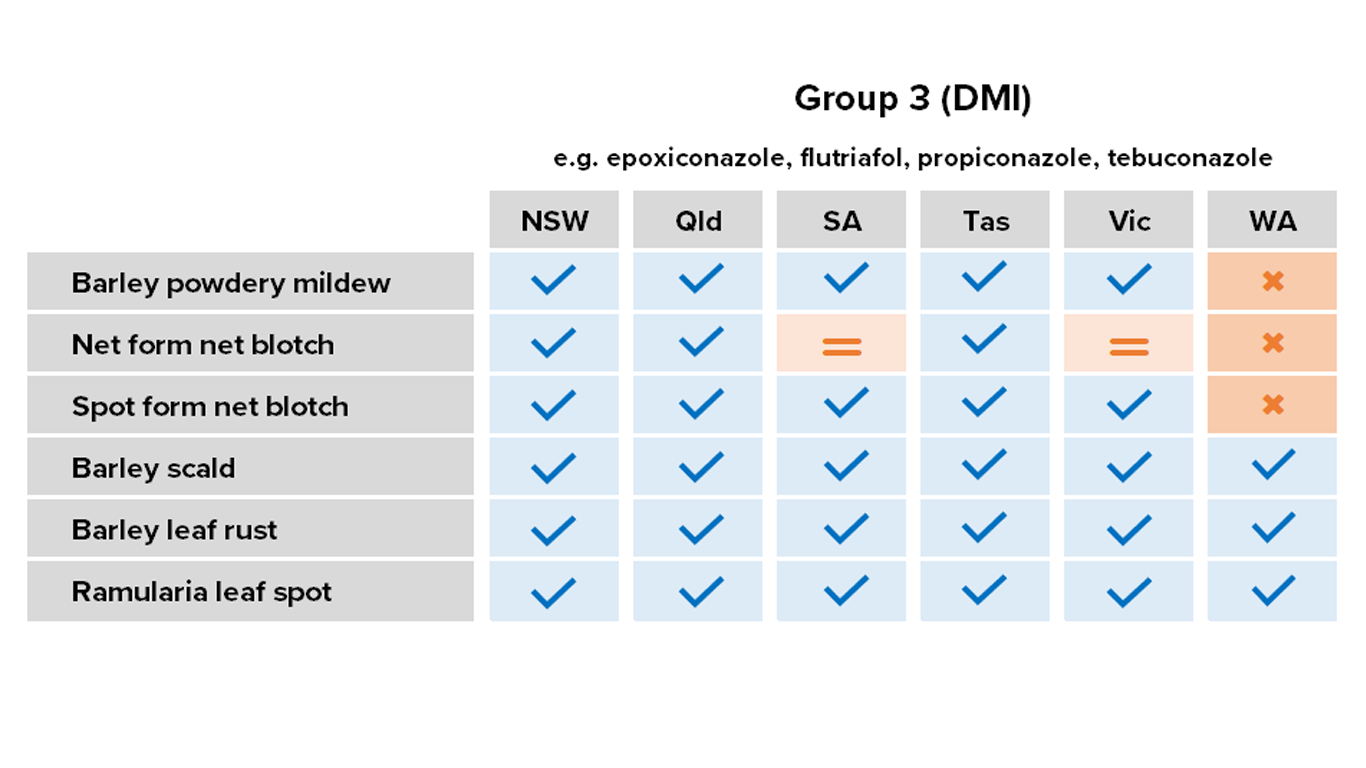
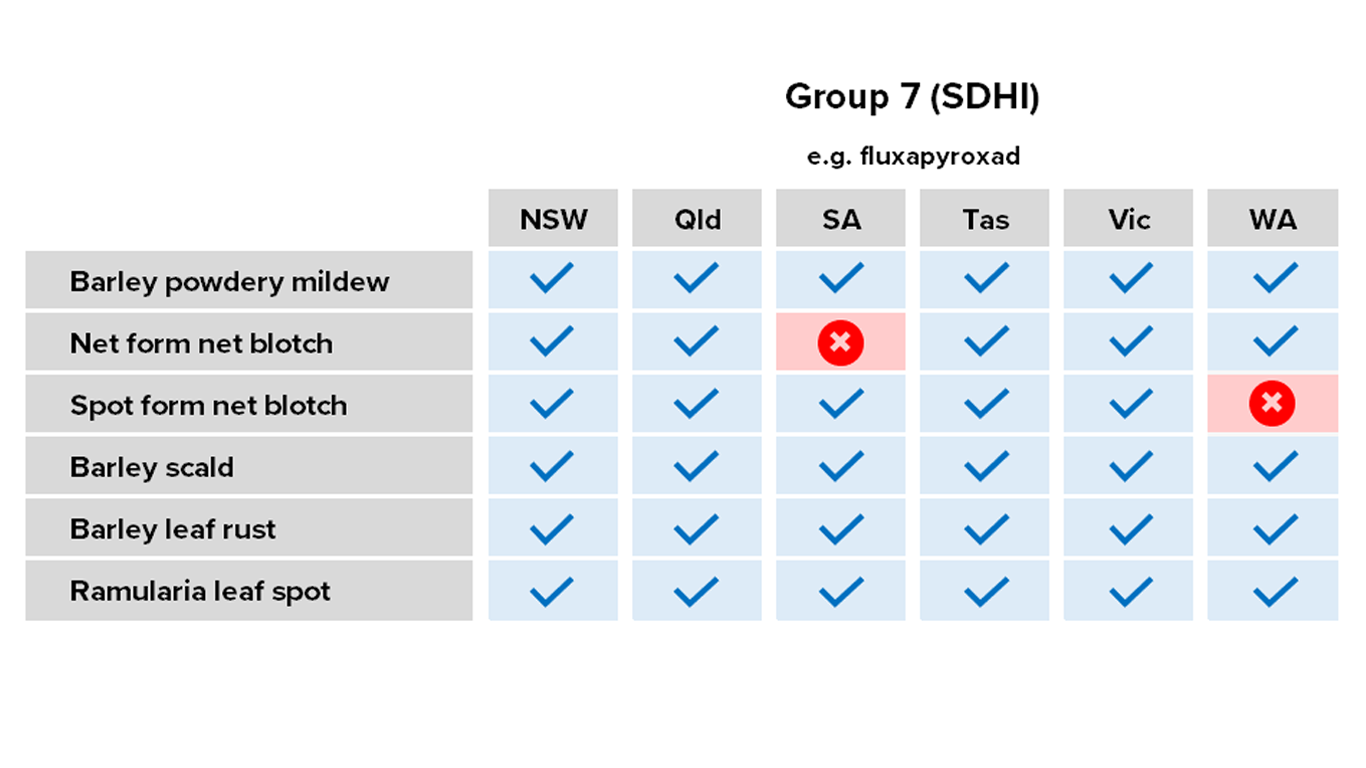
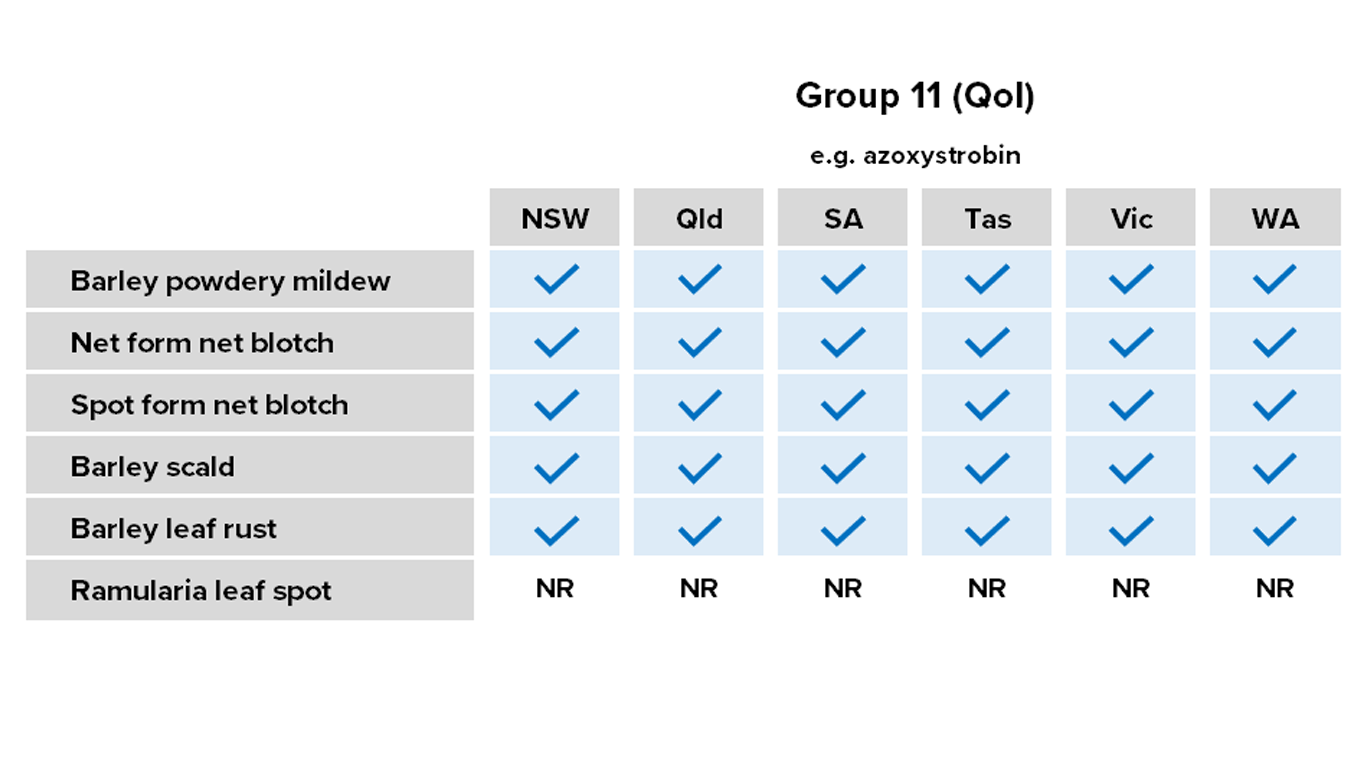
Barley
Fungicides – current field performance
Note – Status of active compounds within each MoA group detailed in each disease section. Farm-level or regional field performance may vary from that recorded here.
✔ Active
= Some active compounds compromised – be selective based on the resistance profile of specific farms or growing regions
🗶 Reduced sensitivity or resistance to some or all active compounds – avoid if possible, or use only in mixture
ⓧ Resistance to most or all active compounds – avoid entirely if possible
NR Not registered for this pathogen
Barley powdery mildew
Blumeria graminis f. sp. hordei

Resistance status
Resistance – Group 3 fungicide tebuconazole in WA.
Reduced sensitivity – Group 3 fungicides propiconazole and flutriafol in WA.
Lab detection – Group 3 fungicides in NSW, Qld, Tas, Vic and WA.
Barley powdery mildew is an important disease of barley, especially in the western and northern regions. It is also potentially very damaging in the southern region in conducive seasons.
Severe infections can occur in winter during both early and later stages of crop growth and can cause significant yield loss in crops with high yield potential.
Barley powdery mildew is typically favoured by susceptible hosts, mild and humid weather (15–22°C, relative humidity > 70%), dense crop canopies, higher nitrogen status, good soil moisture profiles, and extended periods of humid and damp canopies.
The pathogen survives on barley stubble and volunteer barley plants, from which spores can be spread by wind.
Note that barley and wheat powdery mildew are caused by different sub-species, so are crop specific.
Net form net blotch (NFNB)
Pyrenophora teres f. teres

Resistance status
Resistance – Group 3 fungicides propiconazole, prothioconazole and tebuconazole in the Esperance and Kwinana West port zones of WA.
Resistance – Group 7 (SDHI) fungicide fluxapyroxad on the Yorke and Eyre Peninsulas and Kybybolite region, SA.
Reduced sensitivity – Group 3 fungicides epoxiconazole, propiconazole, prothioconazole and tebuconazole in SA and WA.
Reduced sensitivity – Group 7 (SDHI) fungicide fluxapyroxad in SA.
Dual resistance/reduced sensitivity – both reduced sensitivity to the Group 3 fungicide tebuconazole and resistance to the Group 7 fungicide fluxapyroxad on the Yorke Peninsula, SA.
Lab detection – dual resistance to both Group 3 fungicide tebuconazole and Group 7 fungicide fluxapyroxad on the Yorke Peninsula, SA.
Net form of net blotch (NFNB) is an important and increasingly frequent disease of barley across all growing regions, especially in medium to high rainfall zones of South Australia and Western Australia.
It is particularly damaging in wetter years, in systems with high inclusion of susceptible barley in rotations, and where barley is sown into barley stubble. Severe infections can cause 20–50% yield loss and significant reduction in grain quality.
NFNB is typically favoured by susceptible hosts, early sowing, mild weather (15–25°C) and extended periods of leaf wetness. It survives between seasons on stubble, volunteer plants and seed.
Spot form net blotch (SFNB)
Pyrenophora teres f. maculata

Resistance status
Resistance – Group 3 (DMI) fungicides epoxiconazole, propiconazole, prothioconazole and tebuconazole in the Albany and Esperance port zones of WA.
Resistance – Group 7 fungicide fluxapyroxad in the Kwinana West port zone of WA.
Reduced sensitivity – Group 3 fungicides epoxiconazole, propiconazole, prothioconazole and tebuconazole in WA.
Reduced sensitivity – Group 7 fungicide fluxapyroxad in the Kwinana West port zone of WA.
Lab detection – dual reduced sensitivity to both the Group 3 fungicide tebuconazole and Group 7 fungicide fluxapyroxad in the Kwinana West port zone of WA.
Spot form of net blotch (SFNB) is an important disease of barley across all growing regions.
It is particularly damaging in wetter years in the southern regions, in early sown crops and systems with high inclusion of barley in rotations, and where barley is sown into barley stubble. Severe infections can cause 10–45% yield loss and significant reduction in grain quality.
SFNB is typically favoured by susceptible hosts, mild weather (15–25°C) and extended periods of leaf wetness. It survives between seasons on stubble.
Wheat
Fungicides – current field performance
Note – Status of active compounds within each MoA group detailed in each disease section. Farm-level or regional field performance may vary from that recorded here.
✔ Active
= Some active compounds compromised – be selective based on the resistance profile of specific farms or growing regions
🗶 Reduced sensitivity or resistance to some or all active compounds – avoid if possible, or use only in mixture
ⓧ Resistance to most or all active compounds – avoid entirely if possible
NR Not registered for this pathogen
Wheat powdery mildew
Blumeria graminis f. sp. tritici

Resistance status
Resistance – all Group 11 (QoI) fungicides in NSW, SA, Tas. and Vic.
Resistance – Group 3 fungicides propiconazole and tebuconazole in NSW and Vic.
Lab detection – Group 3 gateway mutation associated with reduced sensitivity detected in NSW, SA, Tas. and Vic.
Wheat powdery mildew is a sporadic and important disease in years with conducive conditions, especially in the southern region.
Wheat powdery mildew is typically favoured by susceptible hosts, early sowing, mild and humid weather (15–22°C, relative humidity > 70%), dense crop canopies, good soil moisture profiles, higher nitrogen status and extended periods of humid and damp canopies.
It is spread predominantly via wind-borne spores, and survives on stubble and volunteer plants.
Note that wheat and barley powdery mildew are caused by different sub-species, so are crop specific.
Septoria tritici blotch (STB)
Zymoseptoria tritici

Resistance status
Reduced sensitivity – Group 3 fungicides cyproconazole, epoxiconazole, flutriafol, propiconazole, tebuconazole and triadimenol in NSW, SA, Tas. and Vic.
Septoria tritici blotch (STB) is an important disease of wheat, particularly in high rainfall areas of the southern region.
It is more common in early sown crops and in wet springs, and is typically favoured by stubble retention, susceptible cultivars, cool, wet weather (15–20°C, relative humidity > 70%), dense crop canopies and extended periods of leaf wetness or dew.
It can cause up to 20% yield loss annually, and much more (> 50%) in conducive years. It survives on stubble.
Canola
Fungicides – current field performance
Note – Status of active compounds within each MoA group detailed in each disease section. Farm-level or regional field performance may vary from that recorded here.
✔ Active
= Some active compounds compromised – be selective based on the resistance profile of specific farms or growing regions
🗶 Reduced sensitivity or resistance to some or all active compounds – avoid if possible, or use only in mixture
ⓧ Resistance to most or all active compounds – avoid entirely if possible
NR Not registered for this pathogen
Blackleg
Leptosphaeria maculans

Resistance status
Reduced sensitivity – Group 3 fungicides flutriafol, fluquinconazole, prothioconazole and tebuconazole for isolates from NSW, SA, Vic. and WA populations. Confirmed via in planta and in vitro assays; field implications remain unclear.
Lab detection – Group 2 fungicide iprodione (although not registered for blackleg) in WA.
Blackleg is the most important and costly disease of canola in Australia, and is widespread in all growing regions.
Blackleg is typically favoured by high intensity canola plantings, high annual rainfall (> 500 mm), high total rainfall in the three months prior to sowing (March, April, May; > 100 mm), susceptible cultivars, and extended periods of leaf wetness (> 48h).
It can cause yield losses of 50–90% in conducive years. It is a stubble-borne disease and spores are spread from stubble remaining from the previous season.
Consult the blackleg management guide or BlacklegCM app to determine individual paddock risk for blackleg.
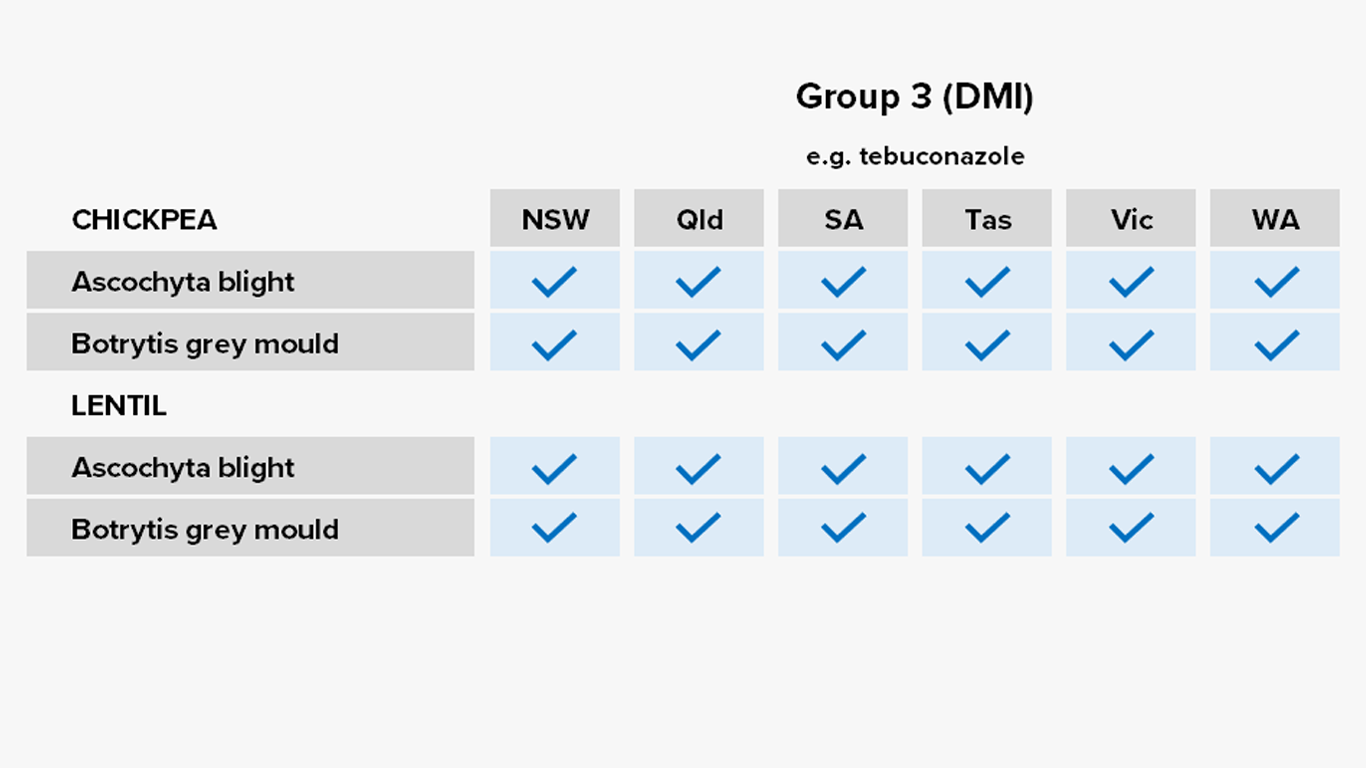
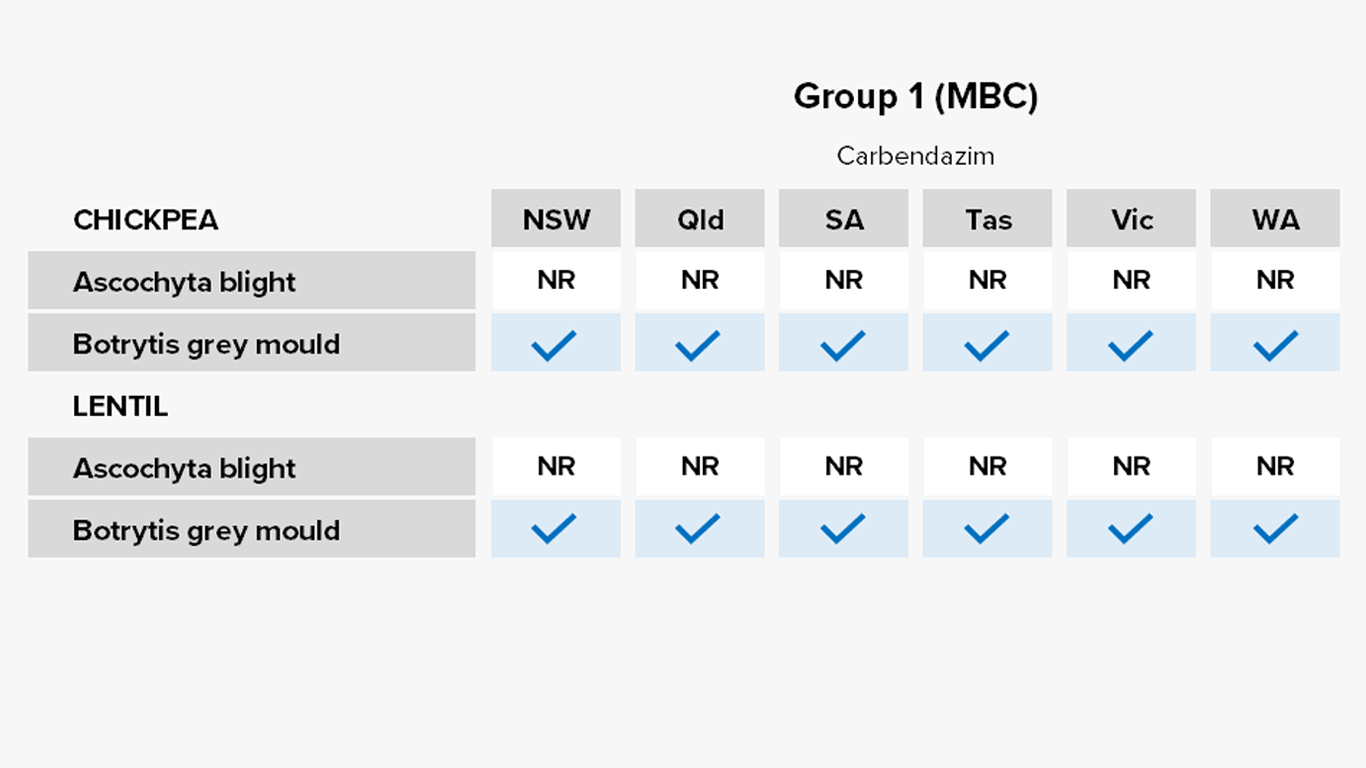
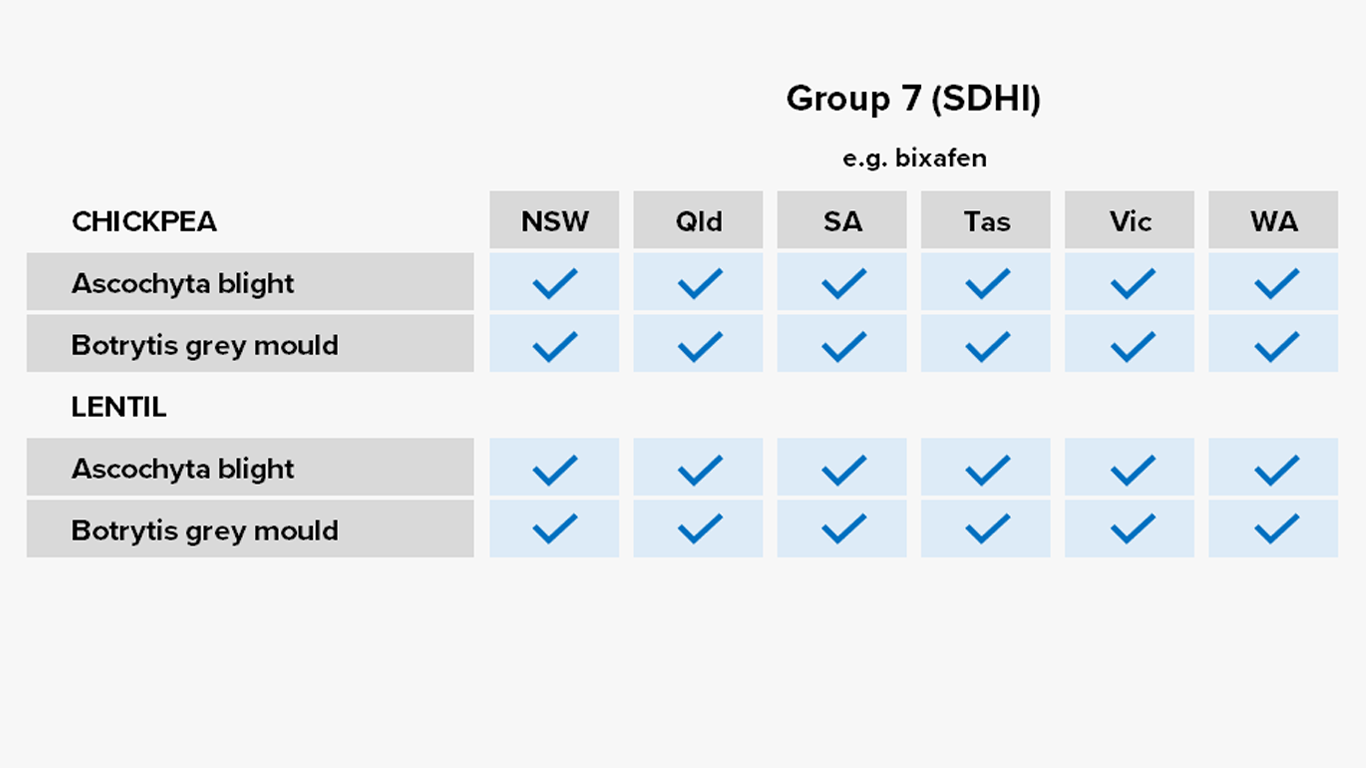
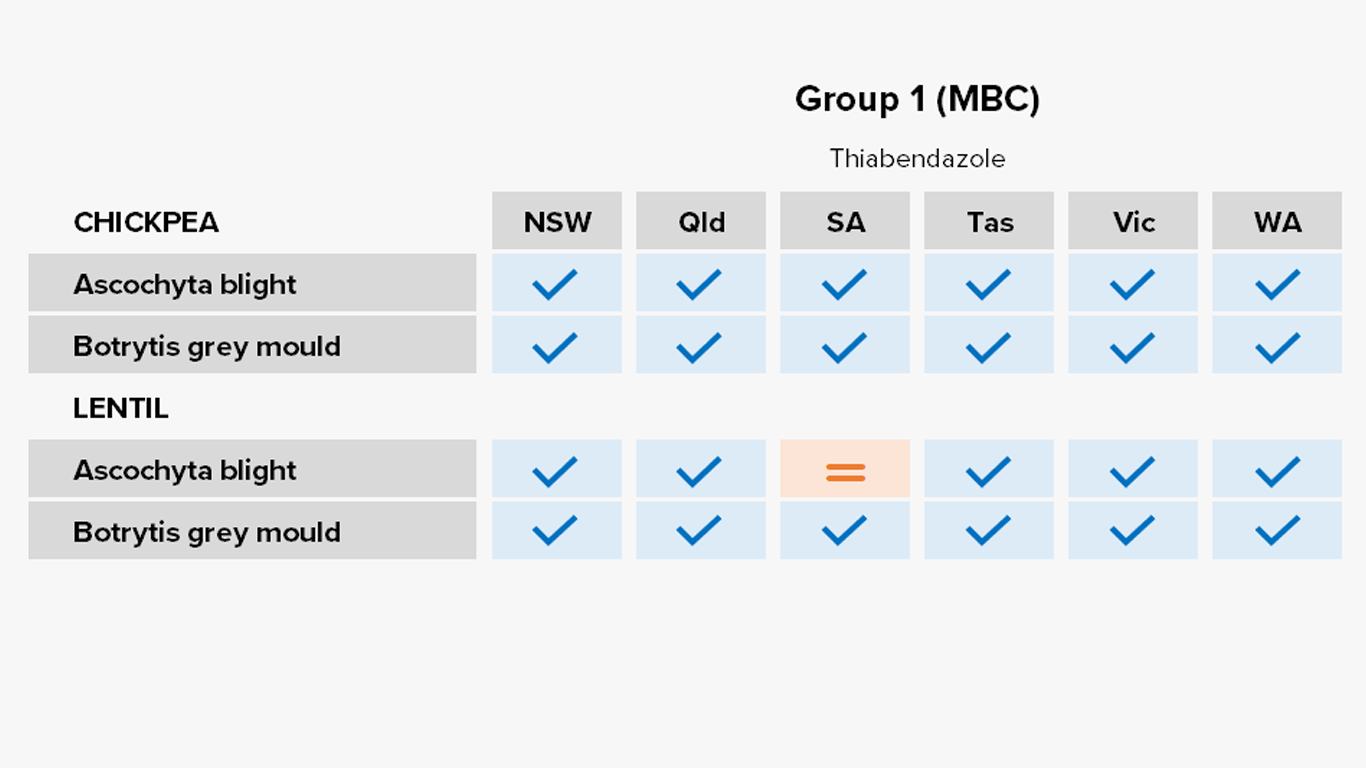
Pulses
Fungicides – current field performance
Note – Status of active compounds within each MoA group detailed in each disease section. Farm-level or regional field performance may vary from that recorded here.
✔ Active
= Some active compounds compromised – be selective based on the resistance profile of specific farms or growing regions
🗶 Reduced sensitivity or resistance to some or all active compounds – avoid if possible, or use only in mixture
ⓧ Resistance to most or all active compounds – avoid entirely if possible
Botrytis grey mould of chickpeas
Botrytis cinerea

Resistance status
Lab detection – Group 1 fungicide carbendazim in SA.
Botrytis grey mould is a serious disease of chickpea, especially in the northern growing regions. Prior to the incursion of Ascochyta blight, it was considered a major disease of chickpea.
It has a wide host range, across grapes and multiple pulse species. This wide host range, combined with its capacity to survive on dead plant material, means inoculum is rarely limiting and infections can proceed quickly when conditions are favourable.
Botrytis grey mould is typically favoured by crops with thick closed canopies that provide conducive temperature and humidity conditions for infection (20–25°C, relative humidity > 90%). Yield reductions can result via seedling loss due to seed-borne root rot, and infection of stems, flowers and leaves throughout the season.
Yield loss in unprotected crops can be as high as 10-25% under conducive conditions, and can cause complete crop failure in extreme cases. It is spread predominantly via airborne spores, infected alternative hosts, and contaminated seed, soil and stubble.
Ascochyta blight of lentils
Ascochyta lentis

Resistance status
Lab detection – Group 1 fungicide carbendazim in SA.
Ascochyta blight of lentils is an important disease of lentils in Australia, especially in the key growing areas of the southern region. It can affect all above-ground plant parts from leaves and stems to flowers and pods, and is often inconspicuous, relying on close inspection to detect it.
It is favoured by prolonged cool and wet conditions (5–15°C) early in the growing season, and heavy rainfall later in the season to establish pod and seed infections.
Unprotected crops can suffer more than 50% yield loss and in severe cases the crop may drop all of its leaves.
Ascochyta blight of lentils is spread via stubble, self-sown plants and seed.